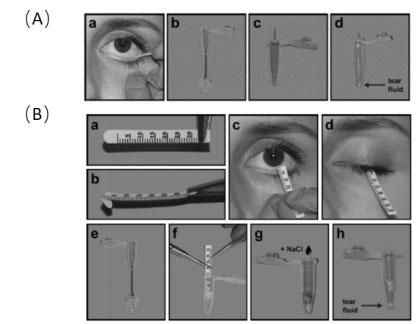
鼎泰集團擁有符合AAALAC标準的非人靈長(cháng)類動物(NHP)飼養設施與一流的生物分析、病理、臨床檢驗和生物标志物實驗室。依托于豐富的NHP疾病模型,專注于代謝、神經(jīng)、心血管、肝腎、眼科及免疫炎症等疾病領域,開(kāi)展具有更高臨床轉化價值的藥效學(xué)/藥代動力學(xué)研究及相關機制研究,鼎泰在提供高品質服務的同時,已與衆多的國(guó)内外藥企、生物技術公司和學(xué)術機構研究建立了值得信賴的合作基礎。

鼎泰集團擁有符合AAALAC标準的非人靈長(cháng)類動物(NHP)飼養設施與一流的生物分析、病理、臨床檢驗和生物标志物實驗室。依托于豐富的NHP疾病模型,專注于代謝、神經(jīng)、心血管、肝腎、眼科及免疫炎症等疾病領域,開(kāi)展具有更高臨床轉化價值的藥效學(xué)/藥代動力學(xué)研究及相關機制研究,鼎泰在提供高品質服務的同時,已與衆多的國(guó)内外藥企、生物技術公司和學(xué)術機構研究建立了值得信賴的合作基礎。


鼎泰集團擁有符合AAALAC标準的非人靈長(cháng)類動物(NHP)飼養設施與一流的生物分析、病理、臨床檢驗和生物标志物實驗室。依托于豐富的NHP疾病模型,專注于代謝、神經(jīng)、心血管、肝腎、眼科及免疫炎症等疾病領域,開(kāi)展具有更高臨床轉化價值的藥效學(xué)/藥代動力學(xué)研究及相關機制研究,鼎泰在提供高品質服務的同時,已與衆多的國(guó)内外藥企、生物技術公司和學(xué)術機構研究建立了值得信賴的合作基礎。


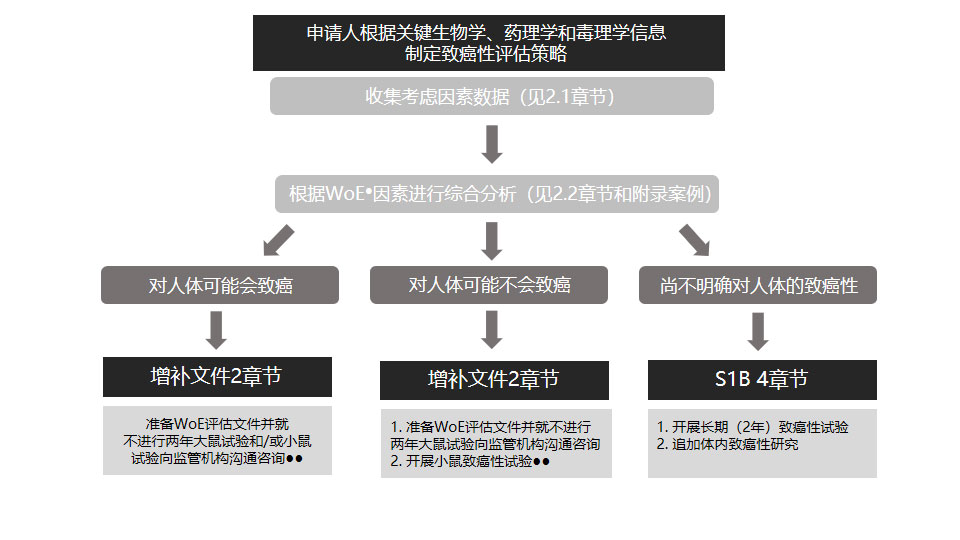
The development of clear and accurate regulatory strategy is crucial in ensuring the quality of R&D and expediting product launches. Drawing from our extensive experience in industrial product development and regulatory evaluation, the Regulatory Strategy Team at TriApex offers clients services in three areas: regulatory strategy, registration application, and technical consulting/training.

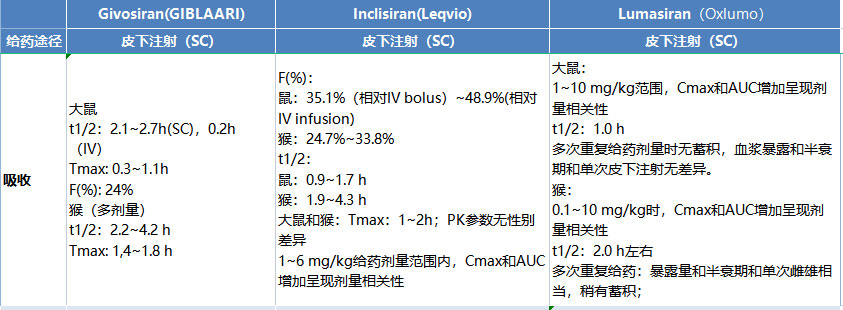
TriApex DMPK team is committed to providing pharmacokinetic research services for PK R&D studies at various research stages, including leading compound discovery and optimization, PCC identification, and other phases, to facilitate drug R&D from non-clinical to clinical stages.

TriApex is a study facility that got GLP certification early by NMPA, where the facility operation and study implementation both comply with NMPA, FDA and OECD GLP regulations. We have rich practical experience in nonclinical safety research, and can conduct various nonclinical studies of different products in various animal species according to regulatory requirements or sponsor demands to efficiently support the clinical development and marketing approval.

TriApex provides our clients medical device services, including development strategies, methodological development and validation, pre-clinical safety assessment, regulatory affairs and quality consultation, and medical writing. TriApex, dedicated to shortening the R&D time, reducing the R&D costs, protecting intellectual property, and promoting successful registration and market approval for our clients, is a high-quality partner of medical device innovation and clinical training.


TriApex, committed to the regulatory thinking throughout the clinical service, has led and participated in hundreds of various clinical trials, covering indications such as oncology, ophthalmology, central nervous system and endocrine diseases.
TriApex provides scientific, regulatory-compliant, high-quality and high-standard services to enable marketing authorization for our sponsors. We have cooperated with more than 100 large pharmaceutical industries and biotechs, maintained friendly cooperative relationships with many domestic clinical research facilities, and established a high quality brand in the industry.

TriApex has established a comprehensive technology platform with advanced equipment and stable infrastructure to provide integrated bioanalysis, biomarker, clinical examination and molecular pathology services.